Support customization and sample delivery!
Cleaning Penicillin Bottles
Author:
Munan Glass
Date:
2025-07-18
Read:
Penicillin bottle has an important application in the field of medicine, often used as a packaging bottle for injection, oral liquid, etc. In order to ensure the safety of drugs, penicillin bottle should be strictly cleaned and sterilized before use to ensure that it is in a sterile state.
Before any penicillin bottle can be reused or filled, thorough cleaning is mandatory. Each bottle is flushed with high-purity water to dissolve residual antibiotics and excipients. Ultrasonic baths then dislodge microscopic particles from the inner walls, ensuring complete removal of biofilm and chemical residue. This step is essential for preventing cross-contamination and maintaining batch-to-batch consistency in pharmaceutical production.

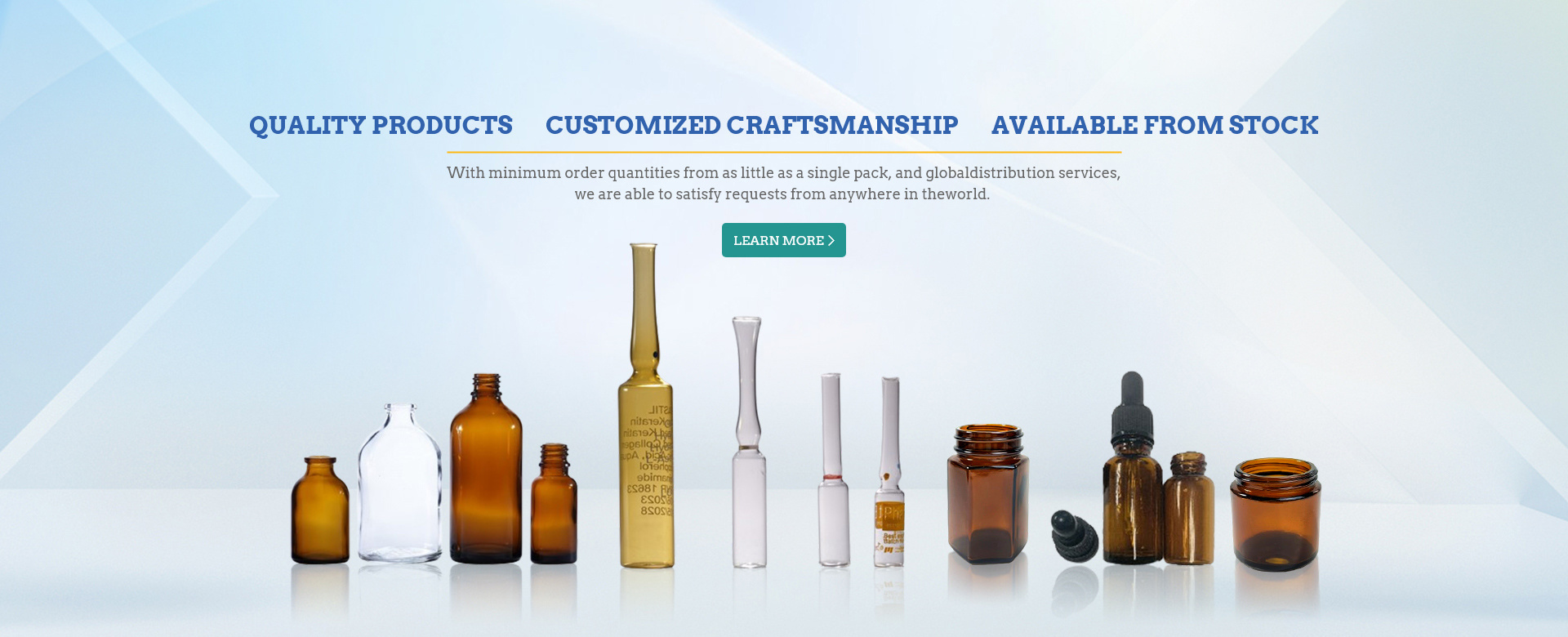
The penicillin bottle, or moulded borosilicate or sodium calcium glass injection bottle, is a small bottle sealed with a rubber plug. Early penicillin was often used in its dressage, hence the name of the bottle. Penicillin bottle has brown, transparent and other types, generally for glass material. The neck part is thin, and the thickness below the neck is the same. The mouth of the bottle is slightly thicker than the neck and slightly thinner than the body of the bottle. It is generally used for vaccines, biological agents, powder injections, freeze-drying, oral liquid and injection, etc. Because penicillin bottles are usually used as oral liquid bottles and injection bottles, they need to be cleaned and sterilized before use, so as to ensure that they are in a sterile state and ensure the safety of drug use.
Sterilization of Penicillin Bottles: Achieving Absolute Safety
After cleaning, sterilization is performed to eliminate all microbial life. Penicillin bottles are commonly exposed to validated dry-heat cycles at 250 °C for 30 minutes or to hydrogen-peroxide plasma for low-temperature sterilization. Both methods meet FDA and EMA guidelines, delivering a sterility assurance level (SAL) of 10⁻⁶. Sterile filtration of incoming air during cooling ensures that no post-sterilization contamination occurs.
The standardization of cleaning and sterilization operation of penicillin bottle has an important impact on the quality of cleaning and sterilization, and directly affects the efficiency of the work, so it is very important to formulate the operating procedures of cleaning and sterilization of penicillin bottle. The cleaning and sterilization operation procedure of penicillin bottle mainly includes four parts: pre-operation confirmation, bottle washing, sterilization and clearance.
The cleaning and sterilization operation of penicillin bottle must be carried out in strict compliance with the operating procedures, to ensure the completion of the cleaning and sterilization work of high quality, to ensure that the penicillin bottle is clean and sterile, to avoid its pollution to drugs, to ensure the safety of drugs.
Related news
Related products
PRODUCT CATEGORY