Support customization and sample delivery!
How to Sterilize Vials for Injection
Author:
Munan Glass
Date:
2025-06-26
Read:
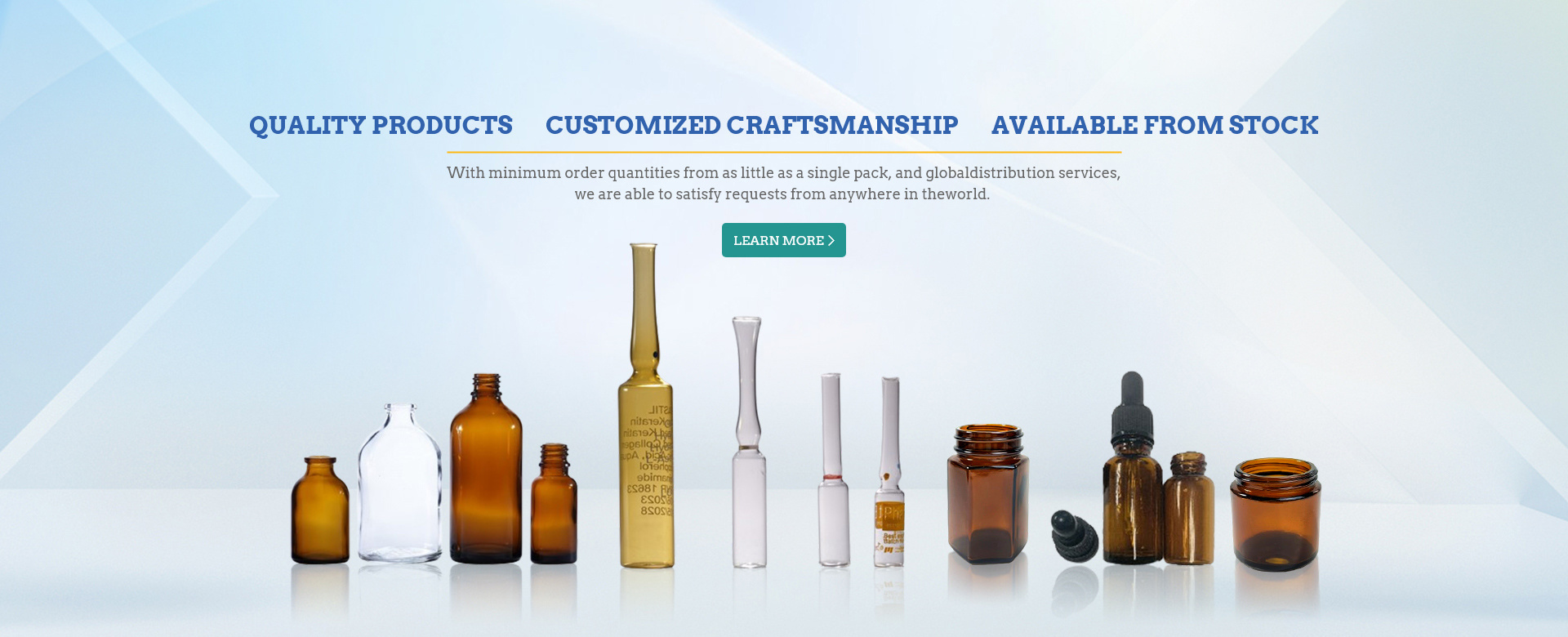
In the pharmaceutical and medical fields, ensuring the sterility of vials for injection is of paramount importance to guarantee patient safety and product efficacy. Among various types of vials, glass vials are widely used due to their excellent chemical stability and protective properties for drug formulations. Chinese primary glass packaging suppliers play a crucial role in providing high - quality glass vials that meet international standards. These suppliers utilize advanced manufacturing processes to produce vials with precise dimensions and high - quality glass compositions, which are essential for effective sterilization and long - term drug storage.

RTU (Ready - To - Use) Vials have gained significant popularity in recent years. They are pre - sterilized and come in aseptic packaging, ready to be filled with drug solutions. The sterilization process for RTU Vials typically involves strict quality control measures in the production environment. Chinese glass packaging suppliers with advanced facilities can produce RTU Vials through methods such as autoclaving or dry - heat sterilization in a controlled cleanroom setting. This ensures that the vials are free from microbial contamination and particulate matter before they reach the end - users, saving time and resources in the drug manufacturing process.
Rubber stoppers are another critical component in the sterilization process of injection vials. They are used to seal the vials and maintain their sterility. Chinese suppliers offer a variety of rubber stoppers made from high - quality materials that can withstand sterilization conditions. The sterilization of rubber stoppers is usually carried out using methods like autoclaving (moist heat sterilization) or ethylene oxide gas sterilization. During autoclaving, the stoppers are exposed to high - temperature steam under pressure for a specific duration to eliminate microorganisms. Ethylene oxide sterilization is suitable for heat - sensitive stoppers, where the stoppers are treated with ethylene oxide gas in a controlled environment to achieve sterility.
Aluminum plastic caps serve as the outer protection for the rubber stoppers and provide an additional barrier against contamination. The sterilization of aluminum plastic caps can be done through dry - heat sterilization or other appropriate methods. Chinese glass packaging suppliers are adept at manufacturing aluminum plastic caps that are compatible with the sterilization processes and can maintain their integrity and functionality after sterilization. The combination of properly sterilized rubber stoppers and aluminum plastic caps with the glass vials creates a secure and sterile packaging system for injectable medications.
When it comes to sterilizing vials for injection, whether they are glass vials from Chinese primary glass packaging suppliers, RTU Vials, or those assembled with rubber stoppers and aluminum plastic caps, it is essential to follow validated sterilization protocols. These protocols should consider factors such as the type of vial and components, the drug formulation, and the required sterility assurance level. By working closely with reputable Chinese glass packaging suppliers and adhering to proper sterilization procedures, healthcare professionals and pharmaceutical manufacturers can ensure the safety and reliability of injectable medications, ultimately contributing to better patient outcomes and the overall quality of healthcare delivery.
Related news
Related products
PRODUCT CATEGORY