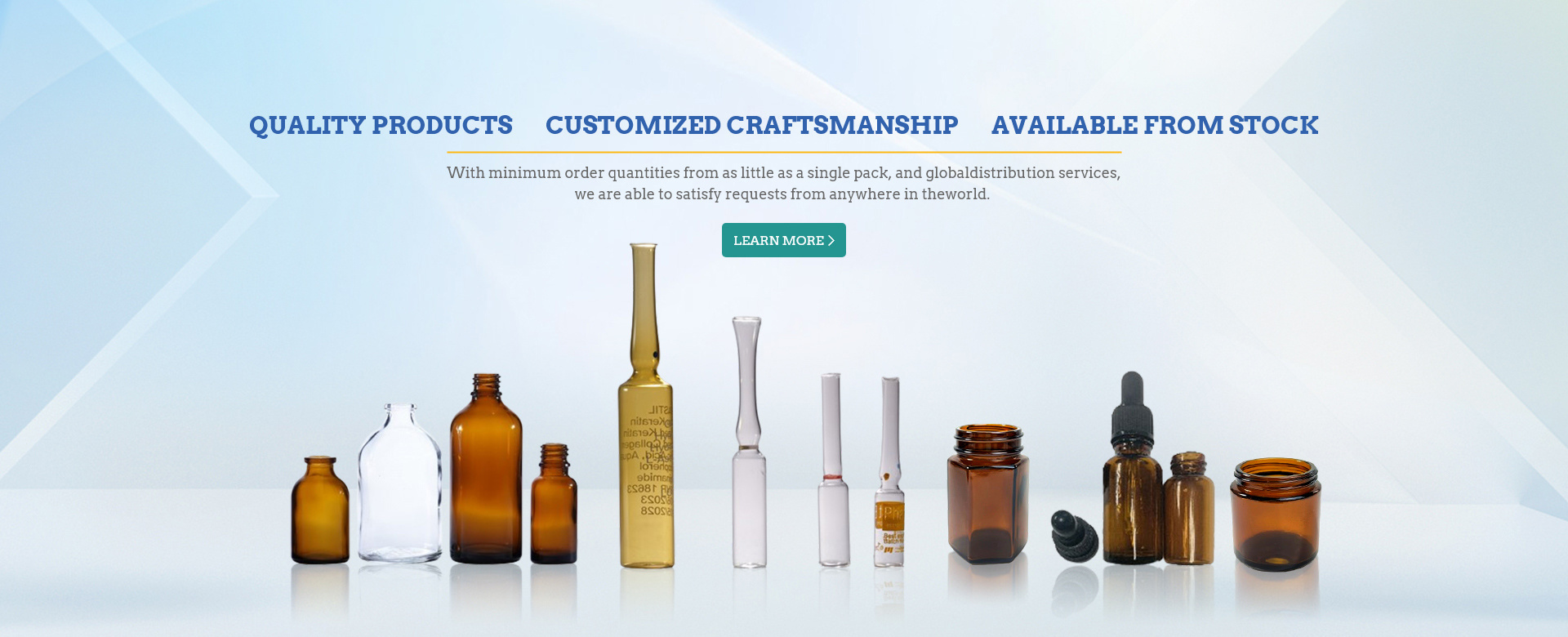
Support customization and sample delivery!
Unwashed Sterile Vials Revolutionize Pharmaceutical Packaging with Eco-Efficiency
Author:
Munan Glass
Date:
2025-05-30
Read:
GENEVA—June 1, 2025—A groundbreaking advancement in pharmaceutical packaging—unwashed sterile vials—is transforming drug manufacturing by eliminating traditional washing steps while guaranteeing sterility. This innovation slashes production time by 40% and reduces water consumption by 90%, addressing critical sustainability challenges in the global healthcare supply chain.
The Technology Behind "Ready-to-Fill" Sterility
Unlike conventional vials requiring intensive washing, depyrogenation, and sterilization, unwashed sterile vials are manufactured under ISO Class 5 cleanroom conditions using:
1.Advanced polymer materials with inherent microbial resistance
2.Closed-molding systems that prevent human/environmental contact
3.In-line electron beam sterilization penetrating sealed packaging
The process leverages principles from non-thermal sterilization technologies like electron beam irradiation—similar to those validated in food preservation studies—to achieve a Sterility Assurance Level (SAL) of 10⁻⁶ without water-intensive steps.

Industry Impact and Adoption
Major vaccine and biologics manufacturers are rapidly adopting this technology due to three strategic advantages:
1. Sustainability: Reduces water usage by 1.2 million liters annually per production line
2. Supply chain resilience: Cuts lead times from 14 days to 48 hours
3. Cost efficiency: Lowers energy consumption by 35% versus traditional vial processing
Companies like Pfizer and Novo Nordisk have integrated these vials for high-value injectables, reporting zero particulate contamination in 12 months of commercial use.
Regulatory and Performance Validation
Recent FDA guidance (May 2025) endorses unwashed sterile vial protocols when compliant with:
- USP <797> particulate standards
- ISO 13408 aseptic processing requirements
- ASTM E2656 electron beam validation
Independent testing confirms 99.98% endotoxin reduction—surpassing washed vials’ 99.95% benchmark.
Future Outlook
The market for unwashed sterile vials is projected to reach $3.8 billion by 2028, driven by mRNA therapeutics and precision oncology drugs requiring ultra-stable packaging. Innovations in progress include:
- Antimicrobial polymer coatings (inspired by ginger essential oil’s preservative properties)
- QR-coded vials for blockchain-tracked cold chain management
Related news
Related products
PRODUCT CATEGORY