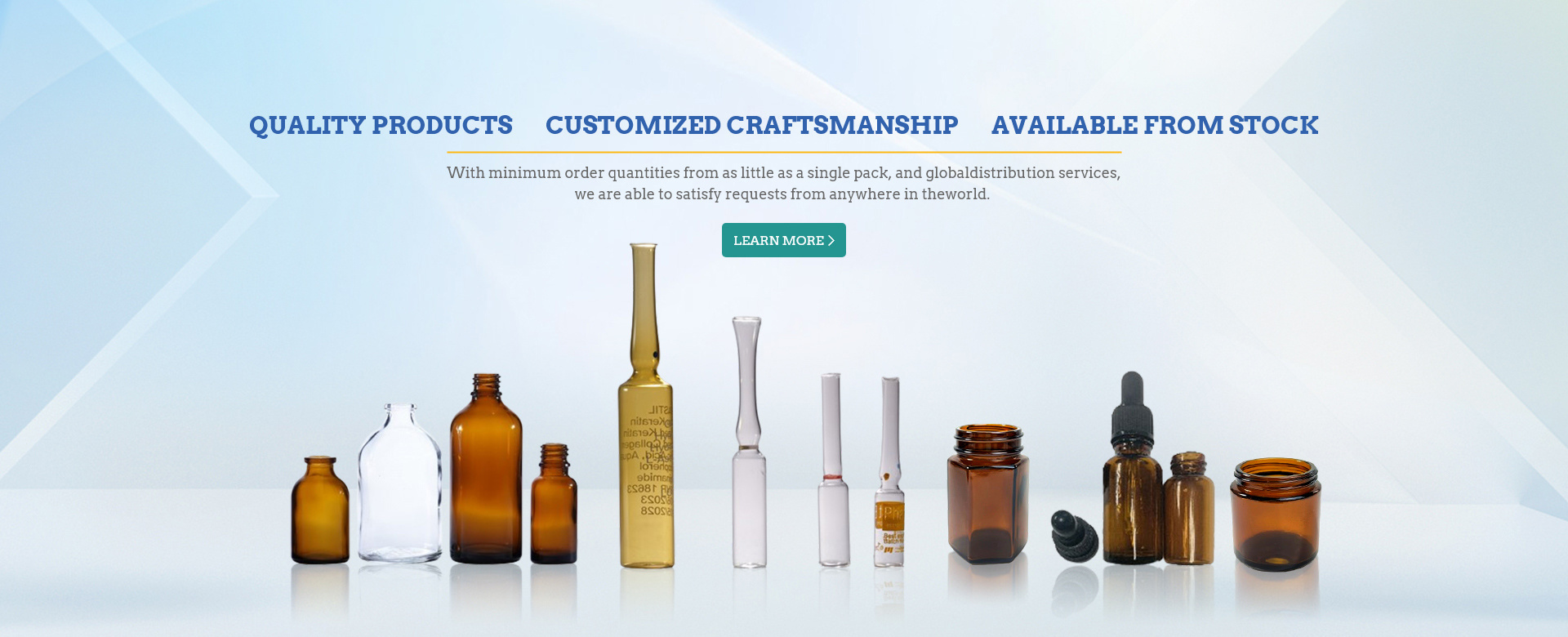
Support customization and sample delivery!
Production process of pharmaceutical glass ampoules
Author:
Munan Glass
Date:
2024-01-24
Read:


Pharmaceutical glass ampoules are critical for storing sterile medications, vaccines, and injectables. Their production demands precision, adherence to quality standards, and compliance with regulatory guidelines. Here's an overview of the 100% original manufacturing process optimized for safety and performance:
1.Raw Material Selection
High-purity Type I borosilicate glass is used due to its exceptional thermal resistance, chemical inertness, and low leaching properties. This ensures compatibility with sensitive pharmaceutical formulations.
2. Glass Tube Formation
Glass tubes are extruded at high temperatures (1,600–1,700°C) using a vertical draw process. Automated systems calibrate tube dimensions (diameter, thickness) to meet exact specifications.
3. Ampoule Molding
Tubes are cut into segments and fed into rotary or linear ampoule-forming machines. A combination of gas flames and molds shapes the neck, body, and tip. The "one-point cut" technique ensures smooth edges to prevent glass particulates.
4.Annealing for Durability
Newly formed ampoules undergo **controlled annealing** in furnaces (500–600°C) to eliminate internal stresses, enhancing mechanical strength and reducing breakage risks during handling.
5.Quality Inspection
Automated vision systems and laser sensors check for defects like cracks, bubbles, or dimensional inconsistencies. Faulty units are rejected instantly to maintain batch integrity.
6.Washing & Sterilization
Ampoules are cleaned with ultra-pure water and air jets, followed by dry-heat sterilization at 250°C+ to achieve **depyrogenation** (endotoxin removal).
7. Sealing & Packaging
Pre-sterilized ampoules are filled in ISO-certified cleanrooms and sealed using high-temperature fusion. Final packaging occurs in nitrogen-flushed environments to prevent contamination.
Airtight ampoules protect sensitive drugs from oxygen, moisture, and microbial ingress. Rigorous testing and sustainable practices (e.g., glass recycling) align with eco-friendly pharmaceutical trends.