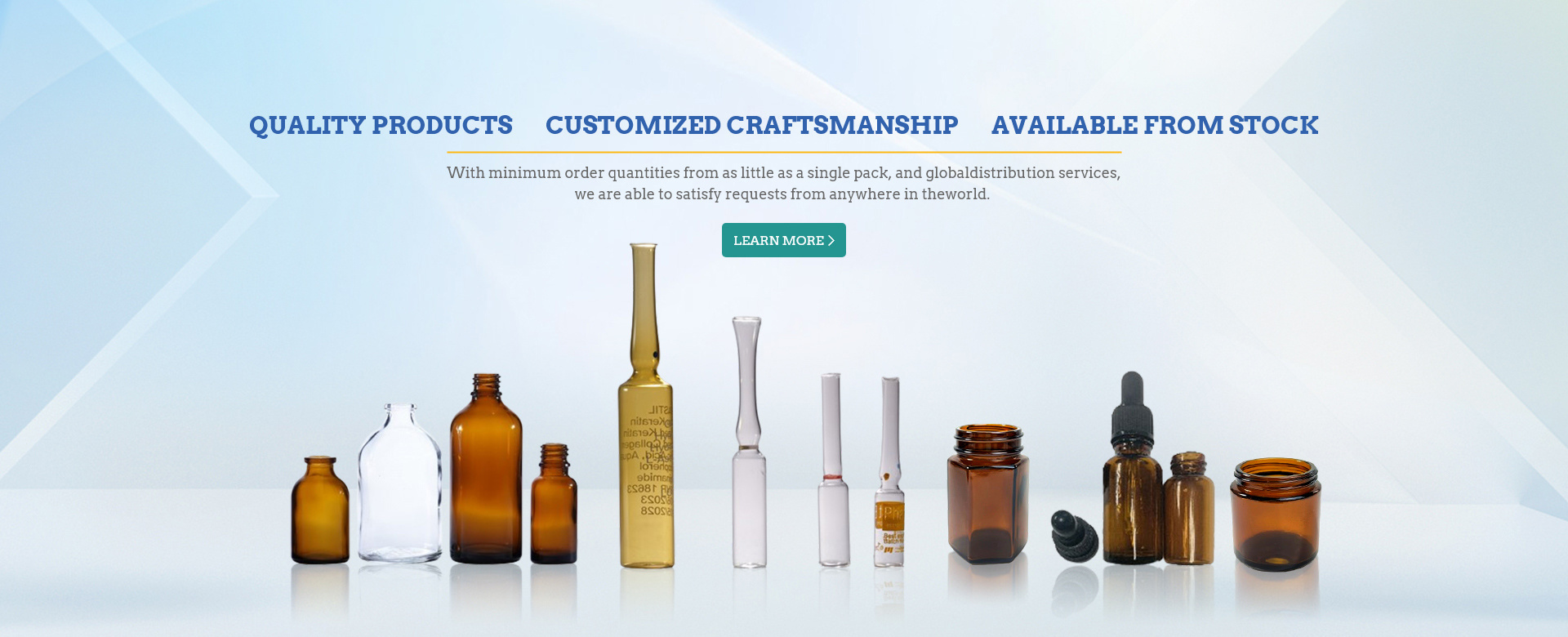
Support customization and sample delivery!
Production process of pharmaceutical glass vials
Author:
Munan Glass
Date:
2024-10-08
Read:


The production of pharmaceutical glass vials is a precision-driven process that ensures safety, durability, and compliance with global healthcare standards. Here’s an overview of the meticulously controlled manufacturing steps:
1. Raw Material Selection
High-purity borosilicate glass is chosen for its thermal resistance, chemical inertness, and low leaching properties. This ensures compatibility with sensitive medications and vaccines.
2. Melting and Forming
The glass raw materials are melted in furnaces at temperatures exceeding 1,600°C. Molten glass is then shaped into vial tubes using either the "blow-and-blow" method (for molded vials) or the "tube-drawing" technique (for tubular vials), ensuring uniform thickness and structural integrity.
3. Annealing
Newly formed vials undergo controlled cooling in annealing ovens to eliminate internal stresses. This step prevents microfractures and enhances mechanical stability.
4. Quality Inspection
Each vial is scrutinized via automated systems for defects like cracks, bubbles, or uneven surfaces. Advanced vision systems and ISO-certified protocols guarantee zero tolerance for imperfections.
5. Surface Treatment
To minimize delamination risks, vials are internally coated with a thin silicone layer or chemically treated for enhanced durability and reduced particle generation.
6. Sterilization and Packaging
Vials are washed, sterilized, and depyrogenated using validated processes. They are then packed in cleanroom environments to maintain sterility until use.
Modern facilities integrate sustainability practices, such as recycling glass cullet and optimizing energy use, aligning with eco-friendly pharmaceutical packaging trends.
By adhering to strict regulatory guidelines (e.g., USP <660>, EP 3.2.1), pharmaceutical glass vial manufacturers deliver products that safeguard drug efficacy and patient safety worldwide.